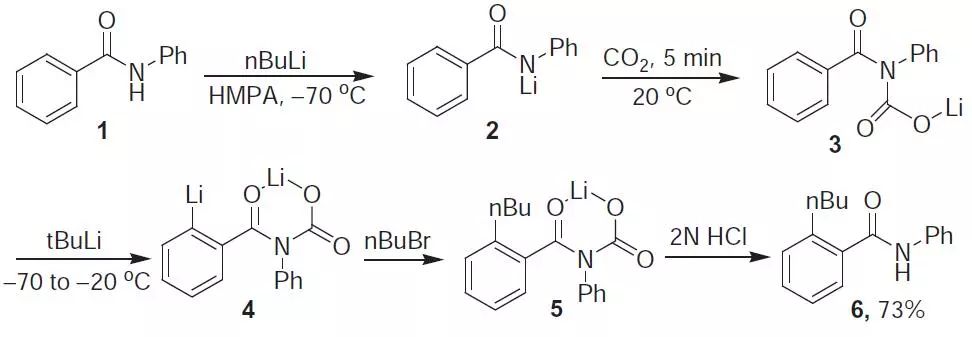
芳基酰胺或芳胺基甲酸酯(主要是N-Boc衍生物)通过和叔丁基锂【Katritzky AR, Org Prep Proced Int., 1987, 19, 263】或仲丁基锂【Snieckus V, Chem Rev., 1990, 90, 879】进行邻位拔氢锂化后进行烷基化的反应。反应中加入LiCl可以加速反应【Collum DB, J Org Chem., 2009, 74, 2231】。
反应操作
2-n-Butylbenzanilide (6). To benzanilide 1 (1.97 g. 10 mmol) in THF (28.5 mL) and HMPA (1.5 mL) was added 2.5 M BuLi (4 mL) dropwise at -70 ℃. The mixture was warmed to r.t. and CO2 was passed through for 5 min. After removal of the solvent under vacuum, THF (30 mL) was added under Ar and 1.7 M t-butyllithium (6.5 mL) was added slowly at -70 ℃. The mixture was
maintained for 20 min at -20℃ and recooled to -70 ℃. n-Butyl bromide 5 (1.37 g, 10 mmol) was added. The mixture was stirred at r.t. for a few hours. The solvent was removed and 2N HCl was added to the residue at 0 ℃. The precipitate was recrystallized to give 1.85 g of 6 (73%), mp 72–73℃.
【Katritzky AR, Org Prep Proced Int., 1987, 19, 263】
相关文献
1 Hauser CR J Heterocycl Chem 1969 6 475
2 Beak P J Org Chem 1977 42 1823
3 Hauser CR J Chem Eng Data 1978 23 183
4 Beak P Acc Chem Res 1982 15 306
5 Katritzky AR Org Prep Proced Int 1987 19 263
6 Schlosser M Tet Lett 1988 29 4277
7 Snieckus V Chem Rev 1990 90 879
8 Hassner A Tet Asymm 2001 12 2269
9 Florio S Org Lett 2005 7 3749
10 Hassine BB Tet Lett 2006 47 6405
11 Collum DB J Org Chem 2009 74 2231
编译自:Organic Syntheses Based On Name Reactions, 3RdEd, A. Hassner, Page 206-207.
2023-2-22 摘自 有机合成公众号