一般酯的氨解通过氨的醇溶液或氨水来进行。氨的醇溶剂氨解反应可通过加入适量的甲醇钠和氰化钠来催化。用氨水直接氨解一般需要加热(当该反应温度到100度时,一定要用高压釜做这一反应),这类反应一般可以通过硫酸铜来进行催化。反应的条件选择主要看酯的活性程度,一般脂肪酸酯的交换要比芳香羧酸酯来得容易,甲酯要比乙酯来得快。对脂肪酸酯,α位的位阻大小也决定了反应的快慢。
酯通过甲酰胺在乙醇钠的存在下,高温也可得到相应的酰胺。这一方法对各类的酯都比较有效,只是产品的分离比直接氨解稍微麻烦一些,但反应较快。
另外近年来,AlMe3-NH4Cl或Me2AlNH2在多官能团及复杂化合物的合成中用的较多,该方法条件较强,各类酯都能很快的氨解。其缺点是AlMe3易自燃,操作不是太方便。
1 氨水用于脂肪羧酸酯氨解示例( J. Chem. Soc. Perkin. Trans. 1; 7,1999, 765-776)
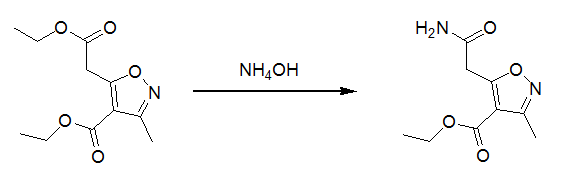
To ethyl 5-ethoxycarbonylmethyl-3-methylisoxazole-4-carboxylate (1.00 g, 4.15 mmol) was added an excess of conc. aqueous ammonia (d = 0.88 kg·dm-3, 5.0 cm3) and EtOH (3.0 cm3), and the suspension was stirred vigorously at room temperature for 14 h. After this period a white solid had precipitated which was filtered and recrystallized (EtOAc) to yield the desired product as a white solid (0.81 g, 92%).
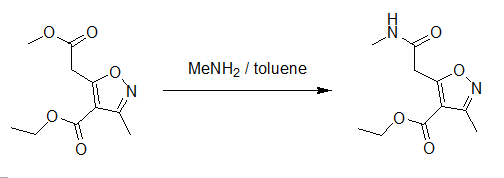
Prepared as described above for ethyl 5-carbamoylmethyl-3-methylisoxazole-4-carboxylate but using methyl-5-ethoxycarbonyl- 3-methylisoxazole-4-carboxylate (1.00 g, 4.15 mmol) and methylamine in toluene (30% w/v, 10.0 cm3), to yield the desired product as a white solid (0.93 g, 99%).
2 氨甲醇氨解脂肪羧酸酯示例(Liebigs Ann., Recl.; 6, 1997, 1165-1172; Bioorg. Med. Chem., 7(3), 1999, 509-516)
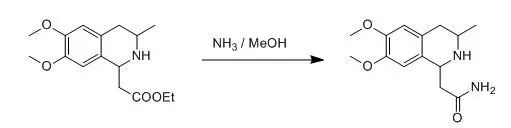
The ester (4.11 g, 14 mmol) was dissolved in absol. methanolic ammonia (100 ml, 20 % NH3), and the solution was allowed to stand at temperature for 3 days, the solvent was then evaporated, and the resulting crystalline was purified by recrystallization.
3 氨水用于芳香羧酸酯氨解示例(Org. Syn., Coll. Vol. 1973,1, 107; 1973,1, 270)
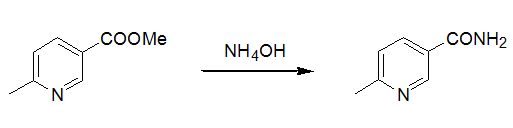
To an autoclave, was added methyl 6-methylniconate (500 g, 3.31 mol), sat. aq. NH4OH (500 ml) and ethanol (500 ml). After sealing, the reaction was heated to 80℃ for 2 days. The cooled reaction mixture was filtrated, and the filter cake was recrystallized to afford white solid (247 g, 54.8%)。
4 HCONH2-NaOEt 体系用于酯氨解示例(EP1522540)
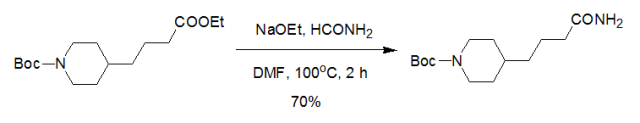
tert-Butyl 4-(3-ethoxycarbonylpropyl)piperidine-1-carboxylate (0.60 g, 2.0 mmol, the product of Production example 43-1) and formamide (0.27 ml, 6.7 mmol) were dissolved in N,N-dimethylformamide (1.0 ml); sodium ethoxide (0.095 g, 1.4 mmol) was added thereto while stirred and heated at 100 .deg.C; the reaction mixture was stirred for 2 hours under nitrogen atmosphere.After cooled to room temperature, the reaction mixture was partitioned between water and ethyl acetate. The organic layer was washed with brine, dried over anhydrous magnesium sulfate, and then the solvent was distilled off under reduced pressure. The residue was purified by silica gel chromatography (eluent; hexane-ethyl acetate = 95:5 to 85:15). The title compound was obtained as a colorless oil (0.38 g, 1.4 mmol, 70percent).
5 NH4Cl-AlMe3 体系用于酯氨解示例(WO2005/12311)
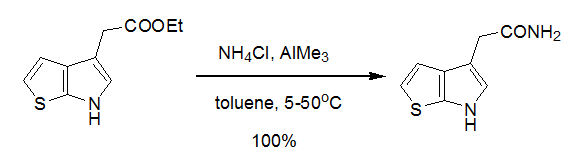
A stirred suspension of ammonium chloride (963 mg, 3 equiv. ) in toluene at 5.deg.C is treated with trimethylaluminium (9 mL of 2 M solution in toluene, 3 equiv.), stirred at room temperature for 2 h, treated with a solution of ethyl 1-(thieno[2,3- b]pyrrol-4-yl) acetate (1.25 g, 6 MMOL, 1 equiv.) in toluene, heated at 50.deg.C for 16 h, cooled to room temperature, quenched with water and extracted with EtOAc. The extracts are combined, dried over MgS04 and concentrated in vacuo to give the title product as a tan oil, 1. 1g (quantitative yield), identified by liquid chromatography and mass spectral analyses.
本文非原创内容,版权归原作者所有,此内容仅限于学习交流之用。
2022-6-2 摘自 有机合成公众号