在有机合成中常见的钯催化偶联反应有:Suzuki-Miyaura偶联, Stille偶联, Negishi偶联, Kumada偶联, Hiyama偶联, Sonogashira偶联, Heck反应, Buchwald-Hartwig反应等等。因此常见的钯催化剂应用非常广泛,虽然这些催化剂都已商业化,但对于大规模生产的反应,可以自己制备降低成本。
Pd(PPh3)4的制备

A mixture of palladium dichloride (17.72 g., 0.10 mole), triphenylphosphine (131 g, 0.50 mole), and 1200 ml of dimethyl sulfoxide is placed in a single-necked, 2L, round-bottomed flask equipped with a magnetic stirring bar and a dual-outlet adapter. A rubber septum and a vacuum-nitrogen system are connected to the outlets, The system is then placed under nitrogen with provision made for pressure relief through a mercury bubbler. The yellow mixture is heated by means of an oil bath with stirring until complete solution occurs (ca. 140 ℃). The bath is then taken away, and the solution is rapidly stirred for approximately 15minutes. Hydrazine hydrate (20 g, 0.40 mole) is then rapidly added over approximately 1 minute from a hypodermic syringe. A vigorous reaction takes place with evolution of nitrogen. The dark solution is then immediately cooled with a water bath; crystallization begins to occur at ca. 125℃. At this point the mixture is allowed to cool without external cooling. After the mixture has reached room temperature it is filtered under nitrogen on a coarse, sintered-glass funnel. The solid is washed successively with two 50 ml. portions of ethanol and two 50 ml portions of ether. The product is dried by passing a slow stream of nitrogen through the funnel overnight. The resulting yellow crystalline product weighs 103.5-108.5 g. (90-94% yield).A melting point determination on a sample in a sealed capillary tube under nitrogen gave a decomposition point of 116℃ (uncorrected). This compares with a similar determination(115℃) performed on the product prepared by the method of Malatesta and Angoletta.
Properties:
The Pd(PPh3)4 complex obtained by this procedure is a yellow, crystalline material possessing moderate solubilities in benzene (50 g/L), methylene chloride, and chloroform.The compound is less soluble in acetone, tetrahydrofuran and acetonitrile. Saturated hydrocarbon solvents give no evidence of solution. Although the complex may be handled in air, it is best stored under nitrogen to ensure its purity.
Pd(PPh3)2Cl2的制备
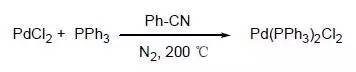
将PdCl2 (50 g, 0.284 mol)和PPh3 (162.5 g, 0.62 mol) 加入苯腈(150 mL)中,氮气置换三次,升温到200℃,反应30 分钟,降温至室温析出晶体,滤出固体,用乙醚洗涤,抽干,得到产品(362 g, 95.8 %)。
Pd(dppf)Cl2的制备
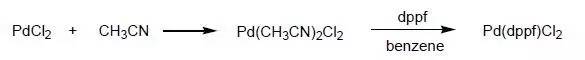
To absolute acetonitrile (1950 mL) degassed via three vacuum/nitrogen ingress cycle was added dichloropalladium (13 g, 73 mmol), the mixture was refluxed at 90~100 °C overnight. The reactant was concentrated to obtain Pd(CH3CN)2Cl2 as orange solid. To a suspension of Pd(CH3CN)2Cl2 (19 g, 73 mmol) in benzene (680 mL) was added a solution of dppf (40.6 g,73 mmol) in benzene (680 mL), the mixture was stirred at rt overnight, the reddish brown precipitate formed was collected by filtration, washed with benzene, and dried in vacuo to give Pd(dppf)2Cl2 (~48 g) in over 90% yield. This is pure enough for the next step, the complex can be recrystallized from chloroform/benzene.
目前市面上常见的,用于偶联反应的钯催化剂种类很多,比如醋酸钯、二苯基磷二茂铁二氯化钯、四三苯基膦钯、二氯二三苯基膦钯、钯碳等等。不同种类的催化剂价格差别非常大,请问它们在催化(偶联)反应方面有什么区别呢?
作者:谢嘉欣
链接:https://www.zhihu.com/question/59214404/answer/162970339
来源:知乎
著作权归作者所有。商业转载请联系作者获得授权,非商业转载请注明出处。
粗略说说,如果只是讨论偶联反应(cross coupling reaction)的话,考虑偶联反应的基本步骤就好了。
一般偶联反应都从零价Pd启动,经历氧化加成(oxidative addition),转金属化(transmetallation),还原消除(reductive elimination)。那么对于各个步骤,不同的配体有不同的表现,比如调整Pd配位环境(配体电性,配体空间体积大小)取决于具体反应要求。
通常富电子配体促进氧化加成,对于难以氧化加成的底物例如氯代芳烃(见Greg Fu的相关工作)有很好的促进反应的作用,而普通的三苯基膦就不行。所以这里二(三叔丁基膦)钯就比四(三苯基膦)钯要好。反过来,缺电子或者大位阻膦配体能促进还原消除,因此对于还原消除很困难或者有beta-H消除竞争的偶联反应可使用这类型配体,还是Greg Fu的例子,sp3-sp3碳还原消除一般很难,但是这类Suzuki反应可以用大位阻配体实现,既减少beta-H消除又因为位阻过大强迫还原消除。
此外,催化剂在反应过程中的稳定性也是重要考量,你不想反应还没开始Pd就死了(Pd黑)对吧?所以配体的存在能稳定零价Pd中间体,使之不聚合成Pd黑析出来。如果只是普通的偶联反应,比如sp2-sp2的Suzuki啊Negishi啊Kumada啊Stille啊sp-sp2的Sonogashira啊诸如此类,只要没有特殊需求,你买的四(三苯基膦)钯和你买的醋酸钯再额外加三苯基膦区别并不大,因为零价Pd可以由二价Pd被膦配体啊胺类有机碱啊还原生成。
其实话说回来,主要还是配体以及pre-catalyst的抗衡离子比较重要,Pd比较次要。但是不同的pre-catalyst有性能差别一般也不好预测。能好好预测的一般只有这个催化剂的Pd是不是正电性(cationic),比如Pd(OTf)2这类带着非配位性抗衡离子的,对于特殊的难以与Pd配位的底物有奇效。其他抗衡离子如Cl有时候又不容易掉下来于是就占着茅坑(配位点)不拉屎(不反应),这时候就需要Ag盐等这类halide scavenger了(偶联反应中这类情况其实很少见,更多见于C-H activation等领域)。但是这类Pd催化剂那么多,具体哪个cationic的Pd催化剂更好,筛了才知道。
补充说明一点,对于C-H活化领域Pd催化剂也是非常常用的,而这时候因为CMD(concerted metallation deprotonation)机理的需求,抗衡离子就多半是羧酸根了,比如常见的醋酸钯(palladium acetate),新戊酸钯(palladium pivalate),三氟乙酸钯(palladium trifluoroacetate)等等。甚至很多情况为了优化反应,采用醋酸钯作为起始催化剂,然后一顿狂筛一遍各种奇葩结构的羧酸的也是见怪不怪,毕竟你没有那么多XX酸钯可以买嘛。。。
总之,如果只是做偶联反应,考虑到使用频率和价格,一般醋酸钯买的很多,其他的常见二价钯啊多多少少能买就买,零价钯比较常见就是dba(二苄叉丙酮)类(如Pd(dba)2和Pd2(dba)3等),膦配体类(如前所述)配合物等,也比较常用,至于好不好用,还是要看反应本身。配体比较重要,所以种类也是尽量越多越好。至于Pd/C,这是非均相催化剂(之前的都是均相催化),基本只用来催化加氢或者脱氢脱卤等。
本文转自网络,版权归原作者所有。
2022-7-18 摘自 有机合成公众号