酰胺是药物研发中非常常见的结构,因此酰胺化反应是最常见的反应之一。小编之前汇总过一些经典的制备酰胺的方法【详见:酰胺的制备汇总】,由于此类转化的重要性,各种新的方法层出不穷,如硼催化,亲氧性过渡金属催化,光催化和硅催化【【酰胺化反应】硅烷类缩合剂----HSi(OCH(CF3)2)3】的酰胺化新方法被报道出来。这些新方法更加注重环境友好度和工艺物质强度(Process Mass Intensity,PMI),来适应医药工业化生产。
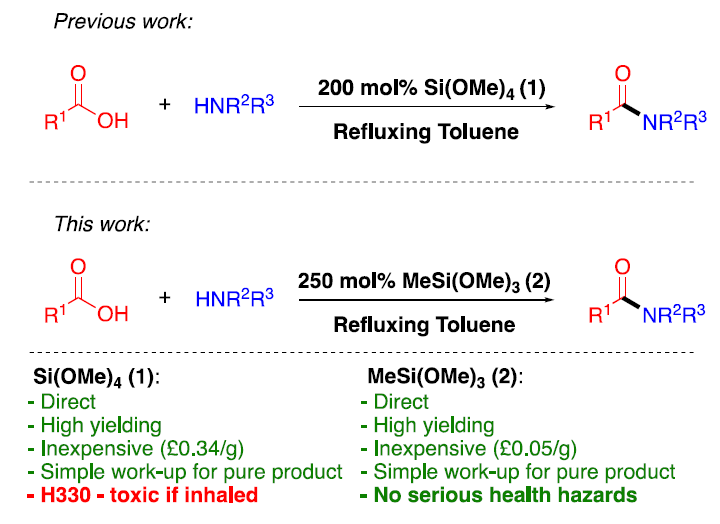
近期伦敦帝国理工学院的Chris Braddock等人,在他们原有工作的基础上开发了一种高效,经济,安全的酸胺缩合试剂----甲基三甲氧基硅烷[MTM, CH3Si(OMe)3] 。酰胺化反应后,仅需要两步简便的后处理即可得到产物,无需进一步纯化。反应后加入碱性水溶液淬灭MTM,然后在反应液中重结晶即可得到产物。此方法具有很高的工艺物质强度,适合工艺生产【Org. Lett. 2022, 24, 1175?1179】。
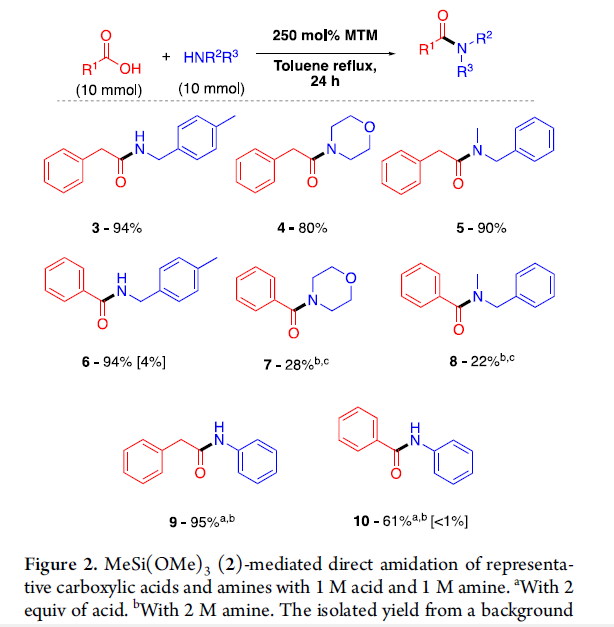
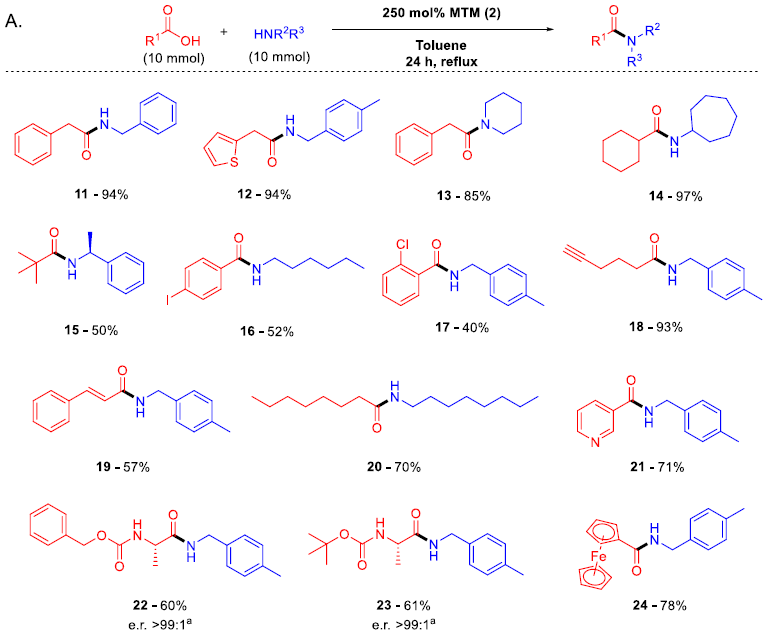
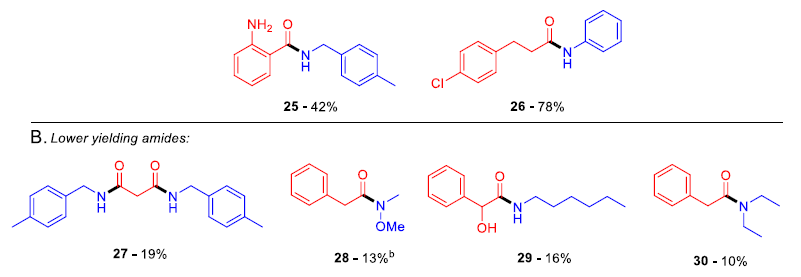
脂肪羧酸和芳香羧酸,脂肪胺和芳香胺都可以利用此反应进行缩合,N-Cbz或N-Boc保护的氨基酸也可以有效缩合,而不发生消旋。此反应主要适合伯胺的酰胺化反应,产物仲酰胺非常适合在反应溶剂甲苯中重结晶,纯化方法十分简便。由于此反应在加热条件下进行,一些低沸点的胺进行缩合产率很低。
反应机理
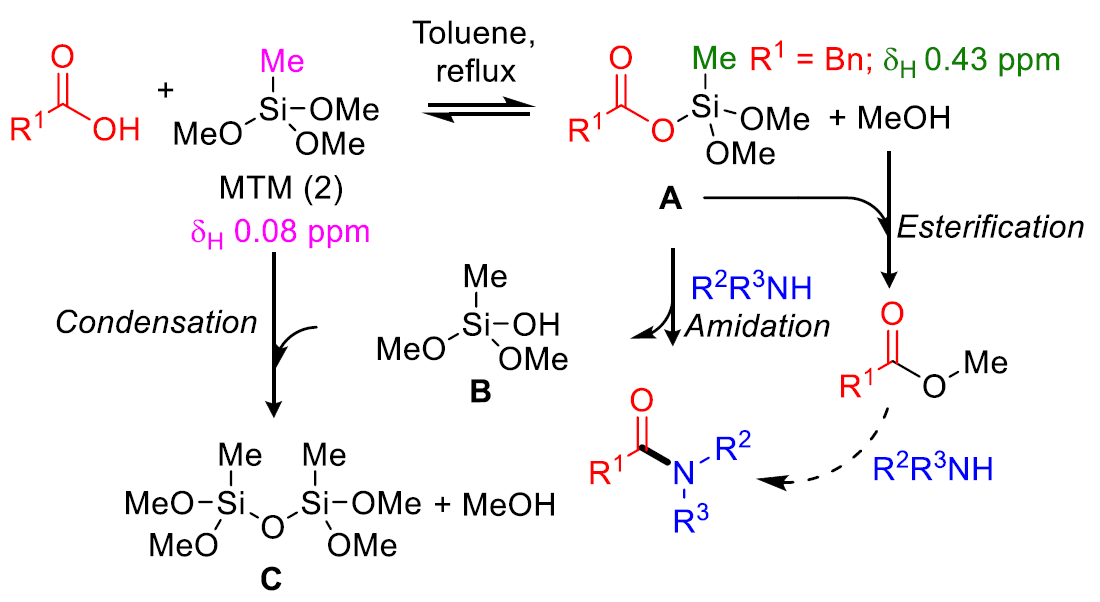
羧酸和MTM加热反应得到酰氧基硅烷中间体(A),胺对中间体A进行加成消除得到产物和硅醇(B),B和另外一分子MTM缩合得到硅醚(C)。因此反应中要加入250mol%的MTM。
反应操作
General Procedure A
Acid (10 mmol, 1 equiv.), amine (10 mmol, 1 equiv.) and MTM 2 (25 mmol, 2.5 equiv.) were heated to reflux in toluene (1 M). After 24 h, the solution was allowed to cool and concentrated in vacuo. The residue was diluted with THF (10 mL) and aqueous NaOH solution (100 mL, 0.3 M) was added with stirring for 1h. Et2O (10 mL) was added, the aqueous phase was saturated with NaCl (s) and the layers were separated. The aqueous layer was extracted with Et2O (35 mL), and the combined organics were washed with aqueous HCl (10 mL, 1 M). The aqueous layer was extracted with Et2O (25 mL) and the combined organics were dried over MgSO4, filtered, and concentrated in vacuo.
General Procedure B
Acid (10 mmol, 1 equiv.), amine (10 mmol, 1 equiv.) and MTM 2 (25 mmol, 2.5 equiv.) were heated toreflux in toluene (1 M). After 24 h, The solution was allowed to cool and the resulting amide precipitate was filtered under vacuum and washed with petroleum ether.
参考资料
Methyltrimethoxysilane (MTM) as a Reagent for Direct Amidation of Carboxylic Acids;D. Christopher Braddock,* Joshua J. Davies, and Paul D. Lickiss;Org. Lett. 2022, 24, 1175-1179.
2022-10-14 摘自 有机合成公众号